Data from CIMA-Q
The CIMA-Q Data and Biological Samples Bank is a structured set of socio-demographic information, results of neuropsychological, neuropsychiatric and clinical assessments (including results of complete blood work) and biological samples from either healthy adults, adults with a cognitive complaint or living with Alzheimer’s. These data have been collected for more than 9 years from the CIMA-Q cohort and are available to researchers interested in Alzheimer’s disease and other related diseases.
Quick downloads
Detailed CIMA-Q Project Protocol (2022-12-15)
Data dictionary (September 2024)
Demographics and evolution of diagnosis (September 2024)
How can I access the CIMA-Q data
| 01. | First you have to be a Consortium for the Early Identification of Alzheimer’s Disease – Quebec member. Form to apply for a membership. |
| 02. | To access the CIMA-Q Bank, please ensure that you have your ethics approval letter for the research project. If your project has not been approved to date you must submit it to the Ageing and Neuroimaging Research Ethics Board (VN REB) for approval (https://staging24.criugm.qc.ca/la-recherche/comite-dethique-de-la-recherche/). The ethics approval number must be provided to the databank manager in order to access the LORIS platform. |
| 03. | Appendix K: Complete the form describing the research project for which you intend to request access. Attach all relevant supporting documents to your application (see list at the end of Appendix K). The User Agreement(s) must be completed and signed by the principal investigator and the administrative representative of the investigator’s institution. Appendices B1 and B3 must be provided for all access applications whereas Appendix B2 must be provided only for biological material access applications. |
| 04. | Submit your documents to the User Access Committee (UAC) at: cimaq@criugm.qc.ca or directly to the Interim UAC Manager, Ms. Andréanne Loiselle |
| 05. | Evaluation of your application by the UAC (evaluation within one week of receiving the complete file). One of the three following statuses might be issued by the UAC following your application:
If your application is accepted with conditions, you will have to respond to the UAC’s requests. Please submit to the following address: andreanne.loiselle@crchudequebec.ulaval.ca |
| 06. | Issuance of the final approval letter for access to the CIMA-Q Data and Biomaterial Bank by the Executive Committee once the user agreements have been signed by the CIUSSS CS MTL administrative manager who oversees the CIMA-Q project. |
| 07. | To access |
| CLINICAL / SOCIO-DEMOGRAPHIC / BIOLOGICAL / NEUROPSYCHOLOGICAL / IMAGING DATA: | |
| |
| BIOMATERIAL | |
| |
| Please note: biological material is reserved for Quebec members only. If you are outside of Quebec, please inquire about the possibility of collaborating with a Quebec researcher. |
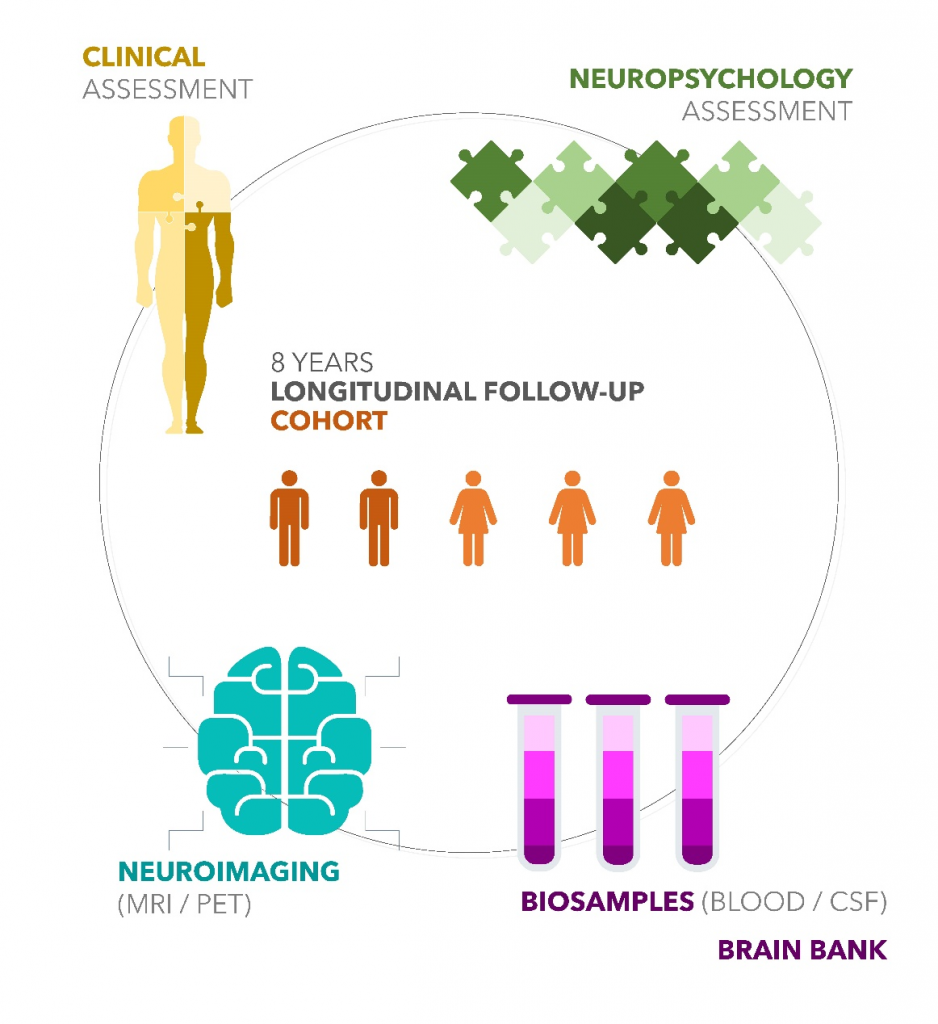
As of July 2023:
PARTICIPANTS: 412 +
H :35.7 % F: 64.3 %
Controls (C): 16.5 %
Subjective cognitive decline (SCD): 43.2 %
Mild cognitive impairment (MCI): 31.6 %
Alzheimer’s Disease (AD): 8.7 %
FULL ASSESSMENT
(Clinical + Neuropsychology + Blood sample)
Initial evaluation: 412 (+241 MRI)
post 2 years follow-up: 184 (+112 MRI)
post 4 years follow-up: 134 (+53 MRI)
post 6 years follow-up: 109 (+58 MRI)
post 8 years follow-up: 23 (+11 MRI)
Full assessment total (Clinical + Neuropsychology + blood collection): 862
Participants with at least 1 complete follow-up after initial evaluation: 222
Participants with 1 follow-up evaluation: 86
Participants with 2 follow-up evaluations: 62
Participants with 3 follow-up evaluations: 54
Participants with 4 follow-up evaluations: 20
NEUROIMAGING
Total PET-scans: 29
Total MRI: 475
Participants with at least 1 MRI: 278
Participants with 1 MRI: 153
Participants with 2 MRI: 78
Participants with 3 MRI: 27
Participants with 4 MRI: 15
Participants with 5 MRI: 5
BIOSAMPLES
Blood 19 000+
CSF (60 participants) 6000+
CSF young controls: to come
BRAIN BANK: 4
All Data and Biomaterial available in the CIMA-Q bank
CLINICAL ASSESSMENT
NURSE AND MEDICAL DOCTOR ASSESSMENT
SOCIO-DEMOGRAPHIC INFORMATION
Nationality, marital status, language, social support network, income, level of education, occupation, retirement, volunteering and activities…
COGNITION
- MoCA (Montreal Cognitive Assessment)
- Telephone-Mini Mental State Examination (T-MMSE)
- Logical Memory test from the Wechsler Memory Scale (short story, immediate and delayed recall)
- Cognitive complaint question (Jessen)
- Clinical diagnosis
COGNITIVE RESERVE
- Cognitive reserve questionnaire (Bartrés score)
- Questions on bilingualism
FUNCTIONAL HABILITIES
- Alzheimer’s disease cooperative study (ADCS) – Activities of Daily Living questionnaire
- Physical self-maintenance scale (PSMS)
- Score – Clinical Dementia Rating (CDR)
NUTRITION
- Mini Nutritional Assessment® SF
GENDER IDENTITY
- Question on gender identity
PSYCHIATRIC SYMPTOMS
- Apathy Inventory (AI)
- Neuropsychiatric Inventory (NPI-Q)
- PHQ-9 (Patient Health Questionnaire)
- Mild Behavioral Impairment Checklist (MBI-C)
CHRONIC PAIN
- Chronic pain Self-Assessment
HEALTH SELF-ASSESSMENT
- Health and well-being survey (SF-36)
- Health status and auto perception of health status
SLEEP
- Chronic insomnia
- Sleep apnea (+ Stop Bang)
- REM sleep disorders
SMELL
- Olfactive capacity test (UPSIT)
VITALS SIGNS AND PHYSICAL MEASUREMENTS
- Resting state blood pressure
- Orthostatic change
- Anthropometric measures
- Grip strength
- Walking speed
MEDICAL HISTORY
- Medical history
- Family history of dementia
- Alcohol and other drug use
- History and evolution: cognitive complaint
- Surgical history
- Personal psychiatric history
- Psychiatric history of the family
- Allergies
NEUROLOGICAL EXAMINATION
- Level of consciousness
- Cranial nerves
- Nervous system
- Cerebellar functions
- Gait
EVALUATION SCALES
- Charlson Score
- Hachinski Ischemic Scale
- Fried frailty index
COMPLETE PHYSICAL EXAM
Including a blood test and a haematological profile
MEDICATION
- List of current medication
COVID-19
- History of infection and vaccination
STUDY PARTNER QUESTIONNAIRES
- Cognitive complaint question (Jessen)
- Neuropsychiatric Inventory (NPI-Q),
- Apathy Inventory (AI)
- Questionnaire relating to activities of daily living (ADCS-PI)
- Mild Behavioral Impairment Checklist (MBI-C)
NEUROPSYCHOLOGICAL, NEUROPSYCHIATRIC AND PSYCHOSOCIAL ASSESSMENT
EPISODIC MEMORY
- Rey Auditory Verbal Learning Task (RAVLT)
- Face-Name memory test (associative episodic memory)
- Cued recall from Memoria
PROSPECTIVE MEMORY
- Envelope Task
SEMANTIC PERCEPTION
- Object Decision test (BORB)
VISUAL DISCRIMINATION
- Visual Perception line orientation test (BORB)
EXECUTIVE FUNCTIONS
- Stroop-D-KEFS (4 conditions)
- Trail making test A and B
- Computerized Hayling task
- Digit Symbol test (WAIS-III)
- Alpha-span (short form)
LANGUAGE
- Verbal fluency (category – animals)
- Boston Naming Test
- Vocabulary test (WAIS-III)
PSYCHIATRIC SYMPTOMS
- Geriatric Depression Scale (GDS-30)
- Geriatric Anxiety Inventory (GAI)
- Apathy inventory (participant)
COGNITIVE COMPLAINT
- Cognitive Change Index (CCI)
- Memory auto-administered questionnaire (QAM, short form)
PSYCHOSOCIAL
- Siegrist’s Questionnaire (effort-reward imbalance at work)
- Karasek’s Questionnaire (Work related stress)
SLEEP
- Insomnia Severity Index (ISI)
- Epworth Sleepiness Scale
- Sleep quality questionnaire
DEMENTIA LITERACY
- Knowledge about Alzheimer’s disease
- Dementia Attitude Scale (DAS)
- Perception Regarding Investigational Screening for Memory in Primary Care (PRISM)
EXPERTISE
- Mobile Device Proficiency
- Technology experience profile
MRI ASSESSMENT
| ANATOMICAL: 3DT1w | |
| PATHOLOGICAL: PD and T2w | |
| VASCULAR: Flair and T2* | |
CONNECTIVITY / FUNCTIONAL:
| |
| And PET SCANS (29 scans) |
BLOOD BIOSAMPLES
- Plasma
- Serum
- Red blood cells
- PBMCs / iPSC / iPSC-derived neurons
- DNA / Buffy coat
- RNA
RESULTS:
- p-Tau 181 / p-Tau 231
- Apo E genotype
- BDNF mRNA (80 participants)
CSF BIOSAMPLES
RESULTS (60 participants):
- Aβ40 / Aβ42 / Aβ38
- Total Tau
BRAIN BANK
4 brains
PLANNED ANALYSIS :
- Thal phases of Aβ deposits
- Braak Scale of neurofibrillary degeneration and Tau
- Detailed vascular pathology
- CERAD score for neuritic plaques
- Braak Scale (α-synuclein/Lewy bodies)
- Protein Transactive Response DNA-binding protein-43 (including LATE)
- Diagnosis
Data collected during the COVID-19 pandemic lockdown
Through a collaboration, the cognitively healthy CIMA-Q participants were interviewed during the first lockdown regarding parameters suspected to be modified by the sanitary measures and the COVID-19 pandemic. The following set of questionnaires assessed COVID-19-related stress, psychiatric symptoms, subjective cognitive complaints, changes in social, cognitive, and physical stimulation, as well as adherence to social distancing recommendations.
- Socio-demographic information
- T-MMSE
- COVID-19 restrictions
- COVID-19 virus
- Social distancing recommandations
- Social network
- Changes in sleep routine
- Sleep habits
- Insomnia Severity Index (ISI)
- Physical activity
- Geriatric Anxiety Inventory (GAI)
- Problems and symptoms related to the pandemic
- Self-perception of health
- Geriatric Depression Scale (GDS-30)
- Question on memory (Jessen)
- Short QAM
- Pain Self-Assessment Questionnaire (short form)
- Alcohol and drug use
 Sylvie Belleville (Ph. D.)
Sylvie Belleville (Ph. D.) Frédéric Calon (Ph. D.)
Frédéric Calon (Ph. D.)  Simon Duchesne (eng., Ph. D.)
Simon Duchesne (eng., Ph. D.) Pierrette Gaudreau (Ph. D.)
Pierrette Gaudreau (Ph. D.) Carol Hudon (Ph. D.)
Carol Hudon (Ph. D.) Marie-Jeanne Kergoat (M.D.)
Marie-Jeanne Kergoat (M.D.) Naguib Mechawar (Ph. D.)
Naguib Mechawar (Ph. D.)